


The research group deals with strategies for the in vitro expansion and optimization of mesenchymal stem cells (MSCs) cultivation. We establish various methods for efficient xeno-free in vitro cultivation of MSCs. Besides conventional culture techniques, such as static two-dimensional (2D) cell culture, three-dimensional (3D) cell culture systems are also developed in our group.
Human MSCs, cell lines and primary cells are cultivated as cell aggregates, on scaffolds and in hydrogels under physiological, in vivo-like conditions. In order to enable complex in vitro 3D systems, physiologically relevant in vitro 3D models for drug screening are generated with the help of bioprinting.
Furthermore, the influences of different in vitro gradients (oxygen concentration, stiffness, growth factors) on the cells in hydrogels are investigated. With the help of this test system, it should be possible to reliably determine the combined influence of various parameters on cell behavior, in order to find optimal culture conditions for the respective application.
Expansion and optimization of mesenchymal stem cells (MSCs) cultivation
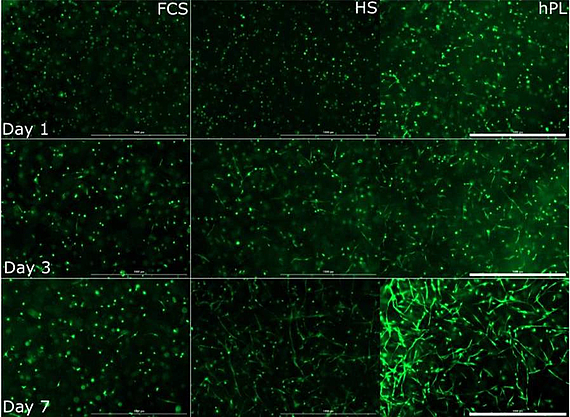
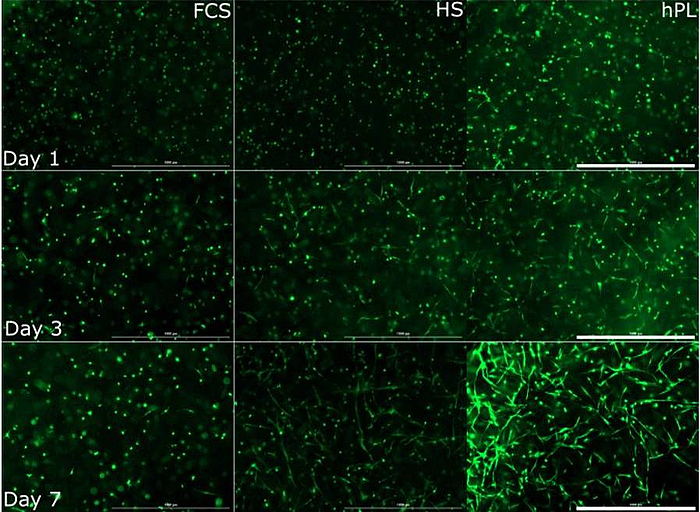
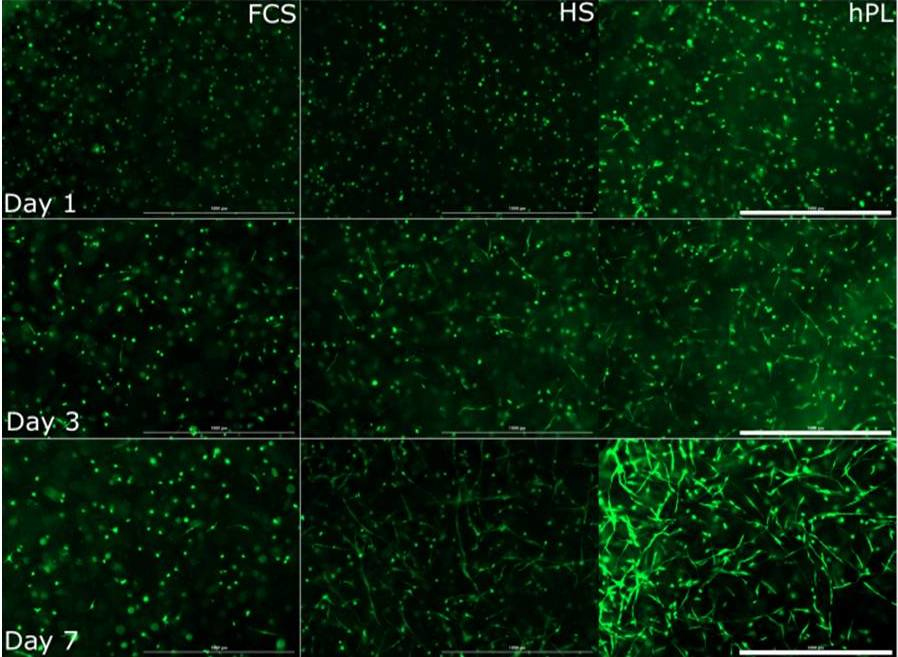
The use of MSCs is promising for cell therapy and tissue engineering. MSCs, isolated from a variety of tissues, are already used in numerous clinical trials. Furthermore, the use of MSCs is less restricted by administrative requirements than the use of embryonic and induced pluripotent stem cells.
Since therapeutic applications typically require several million MSCs per patient, it is necessary to develop optimized culture protocols for in vitro cell expansion. In our projects we use MSCs which are obtained from adipose tissue in cooperation with the Department of Plastic and Reconstructive Surgery of the Hannover Medical School (MHH). Together with the Department of Research and Development of the German Red Cross, platelet lysates are evaluated as supplements for in vitro MSC cultivation and compared with traditional protocols (with FCS and human serum).
Gradient hydrogels
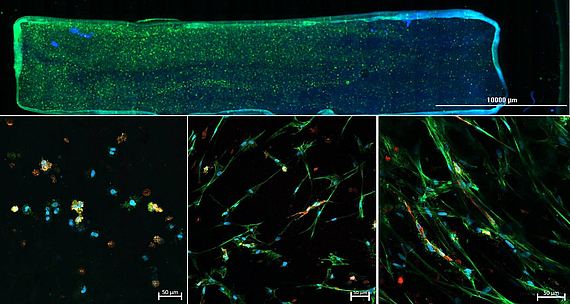
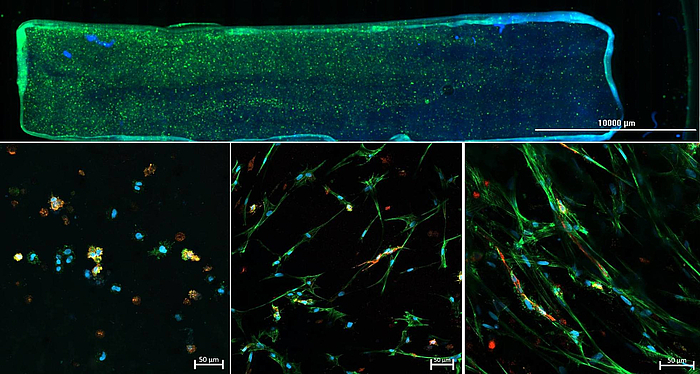
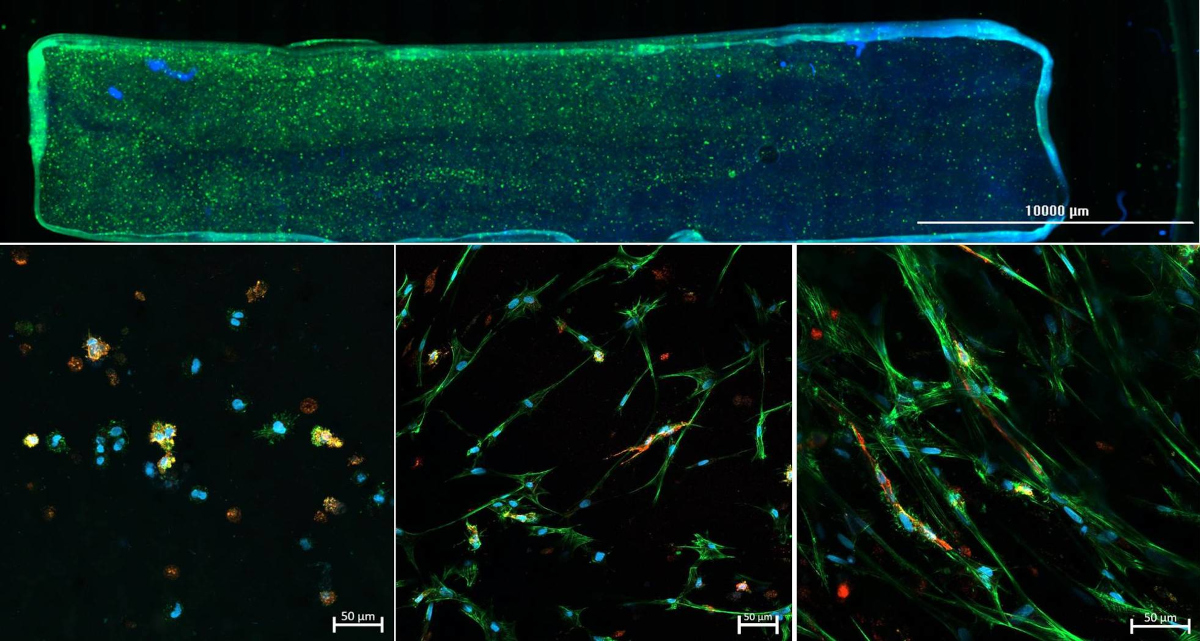
As part of the DFG-funded project "3D Two Gradient Systems for Functional Cell Testing" (398007461), we are developing hydrogel-based in vitro gradients. The aim of the present research project is the development of a 3D cell culture system where multiple-gradient hydrogels can be created in order to obtain complex, but precisely defined in vitro microenvironments. Through variation of the hydrogel composition, mechanical gradients can be realized in the culture model, which allow a systematic investigation of the influence of microenvironmental conditions. Orthogonally, an oxygen concentration gradient is established, so that combinations of hydrogel composition and different oxygen concentrations can be tested within one experiment. With the help of this novel test system, it should be possible to quickly and reliably determine the combined influence of various factors on cell behavior, in order to find optimal culture conditions for specific applications.
Hypoxia reporter cells
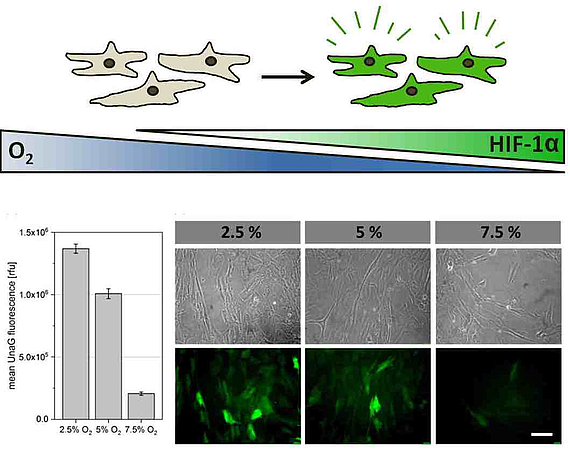
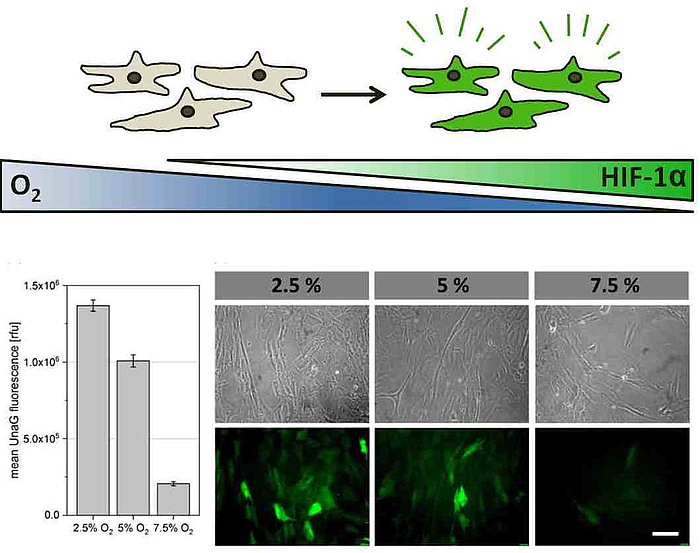
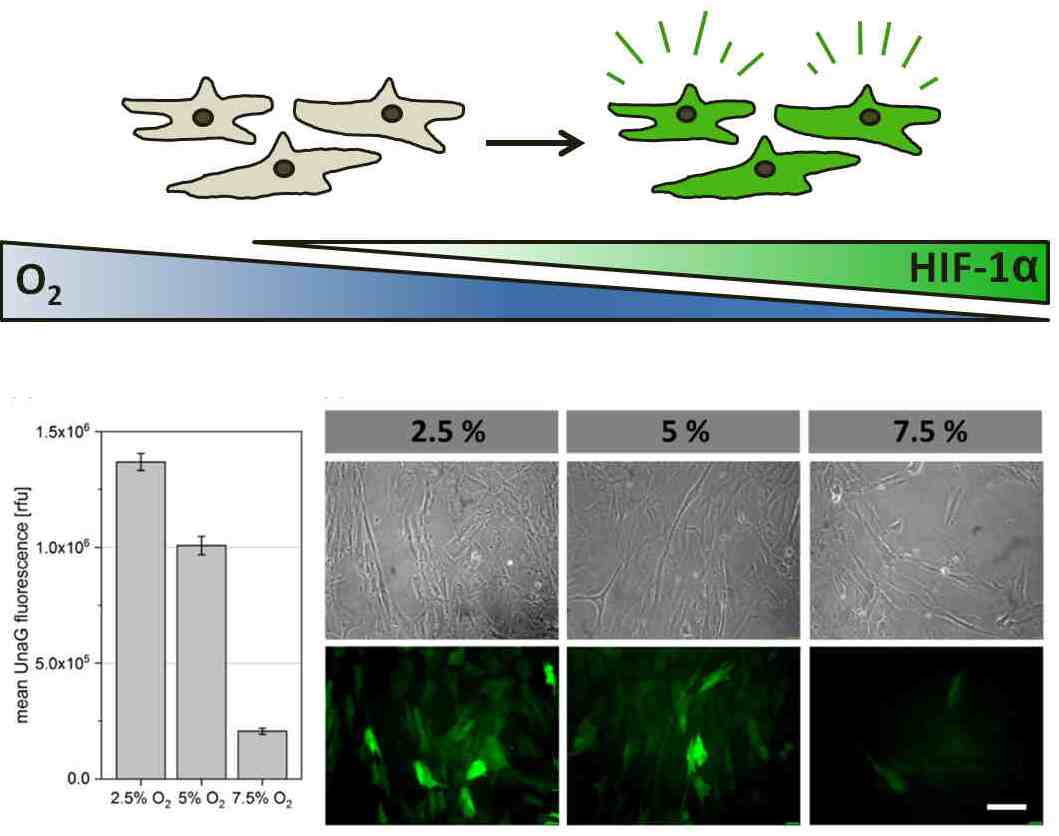
The implementation of clinically relevant reporter cells serves as a valuable instrument for the spatiotemporal recording of the restriction in oxygen supply in various in vitro systems. In cooperation with AG Beluosov (Laboratory for Molecular Technologies, Shemyakin-Ovchinnikov Institute for Bioorganic Chemistry, Moscow), various cell types (MSCs, chondrocytes, endothelial cells etc.) are modified with genetically programmed hypoxia sensors and their hypoxic reactions ("hypoxic signatures") in various 3D cultivation systems (cell aggregates, hydrogels and scaffolds) are evaluated. Overall, the project aims to provide valuable information about the occurrence of hypoxia in 3D in vitro systems and the reactions of different human cells. The different reporter cells, created in this project also represent an innovative toolkit for other areas of application, for example in the area of bioprinting, the characterization of biomaterials or the modeling of diseases.
In vitro Cultivated Fat
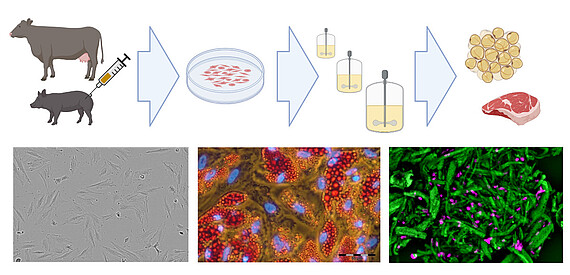
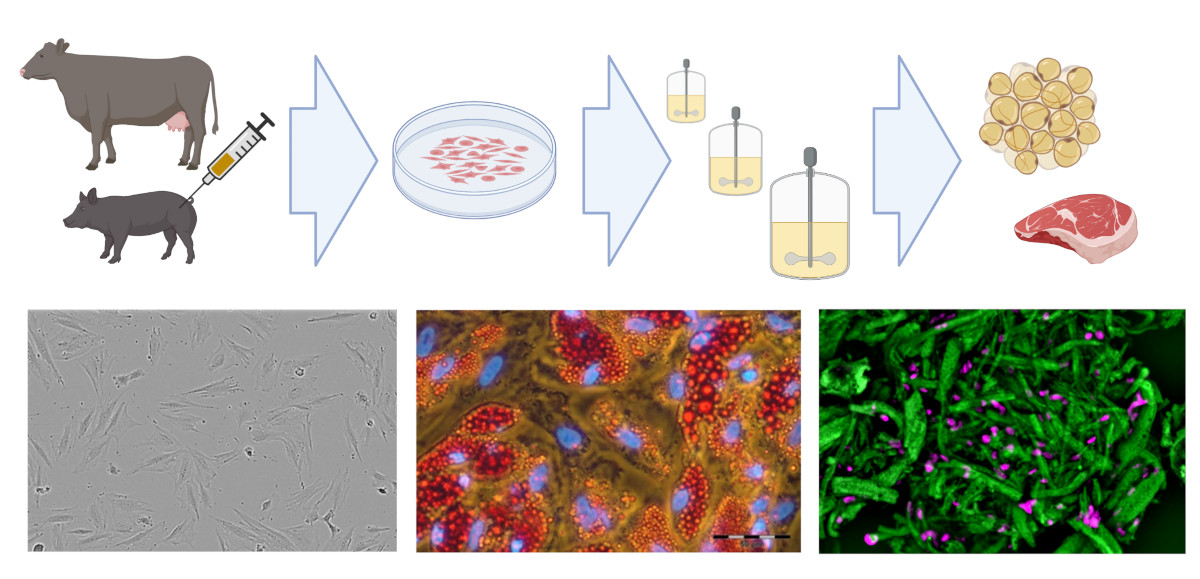
Existing know-how in mammalian cell cultivation (including cultivation of mesenchymal stem cells) can also be transferred to the rapidly growing field of novel foods, especially to cellular agriculture. Here, cells isolated from agronomically important species are expanded and differentiated ex vivo to produce either cell biomass for unstructured meat or cells growing in scaffolds for structured meat analogs. In cooperation with the start-up Cultimate Foods from Berlin/Göttingen, our working group is developing bioprocesses for the isolation, expansion and differentiation of anchorage-dependent porcine and bovine cells for the production of cell-cultured fat. The manufacturing of cultured fat for food is challenging as it requires a cost-effective process that includes only food-grade cell culture components. Nevertheless, there are already first promising results that give the technology great hope.
Contact


30167 Hannover




